COVID-19 nucleic acid detection raw material solution: fast, freeze-dried
Currently, the SARS-CoV-2 coronavirus that causes the COVID-19 pandemic has mutated over time, spreading faster and more stealthily. The prevention and control of pandemics should be deeply concerned in this critical situation. Yeasen Biotechnology (Shanghai) Co., Ltd. continuously provides customers with high-quality and stable nucleic acid detection raw materials to speed up and simplify COVID-19 nucleic acid testing.
1. What is the method to quickly complete nucleic acid detection?
2. Quickly complete the product matching of nucleic acid detection
3. What are the ways to reduce the cost of nucleic acid testing?
4. Product matching to reduce the cost of nucleic acid testing
1. What is the method to quickly complete nucleic acid detection?
At present, the new crown epidemic is swept across the world, and the use of in vitro diagnostic nucleic acid detection methods is still an indispensable means for how to confirm the infection. In vitro diagnosis refers to products and services that obtain clinical diagnostic information by testing human samples (blood, body fluids, tissues, etc.) outside the human body, and then judge diseases or body functions. One of the key steps in the detection of human samples is the purification of nucleic acids (DNA and RNA) in the samples. The method of nucleic acid purification is an important factor affecting the quality of the extracted nucleic acid, and only high-quality nucleic acid can satisfy various downstream applications. Nucleic acid is the basis of molecular biology, and nucleic acid extraction is a threshold for nucleic acid detection, that even the entire molecular industry cannot bypass. In many cases, the quality of nucleic acid extraction from a sample directly determines the validity of the test results.
Nucleic acid extraction provides answers to a wide range of studies and applications (eg: cloning, qRT-PCR, and next-generation sequencing technologies in the whole genome, transcriptome category), and the nucleic acid obtained can be used in a variety of ways. The principles and requirements for accounting, extraction, and purification are as follows: ensure the integrity of the primary structure of nucleic acid; exclude the contamination of other molecules; there are no organic solvents and high concentrations of metal ions that inhibit enzymes in nucleic acid samples; other biological macromolecules, For example, the contamination of proteins, polysaccharides, and lipid molecules should be minimized; the contamination of other nucleic acid molecules should be excluded, such as DNA should be removed when extracting RNA molecules, and vice versa.
One-step RNA technology is a simple, fast, and safe nucleic acid extraction solution. It can not only provide a complete nucleic acid extraction + amplification solution but also improve detection efficiency. The extraction technique is divided into two steps: sample lysis and nucleic acid purification. The first step is sample lysis. Common sample lysis techniques require heating. Such as physical heating cleavage, proteinase K cleavage, etc., may lead to a series of problems such as aerosol pollution. The one-step RNA technology does not require heating and boiling, which is simple, fast, and more economical. The second step is nucleic acid purification. With the development of in vitro diagnostic industry technology, from the initial gradient centrifugation method, and silica gel column method to the latter magnetic bead method. The loss of nucleic acid is gradually reduced, the steps are simplified, and the purity and efficiency of nucleic acid purification are improved. Due to the technical advantages of the first step of sample lysis, the one-step RNA technology can completely omit this step and avoid purification.
The nucleic acid releasing agent can lyse the cell or viral protein coat, release the nucleic acid in it, and then perform nucleic acid detection by PCR experiment. There are different types of nucleic acid release agents, among which inactivated virus preservation solution is a commonly used nucleic acid release agent in new crown detection. The most fundamental feature of this nucleic acid release agent is that it can rapidly inactivate the virus and degrade the viral protein membrane capsid. Then the viral nucleic acid (COVID-19 is RNA) is released so that it can be used for PCR amplification experiments after retrograde reverse transcription to achieve the purpose of using nucleic acid to detect the virus.
2. Quickly complete the product matching of nucleic acid detection
PCR is a molecular biology technique used to amplify specific nucleic acid fragments. Its biggest feature is that it can enrich and increase trace amounts of nucleic acids in a large amount, to facilitate the detection of trace amounts of nucleic acids. PCR methods commonly used in medical diagnosis are mainly real-time quantitative PCR (qPCR) methods based on dual fluorescent probes. The objects of in vitro diagnosis using the qPCR method include human genomic DNA, DNA virus, RNA virus, bacteria, fungi, and so on. Due to the single-stranded structure of RNA, which is unstable and easily degraded, the requirements for sample processing are quite high. Amplified RNA samples need to be pre-treated and nucleic acid extracted using more complex methods, followed by purification. Only after obtaining pure nucleic acid can stable results be obtained.
Product collocation: Nucleic Acid Release Reagent (Cat#13720ES) (Inquire), Hieff Unicon™ Fast Multiplex One Step RT-qPCR Probe Kit(UDG plus) (Cat#13660ES)(Inquire).
2.1 Nucleic Acid Release Reagent (13720ES)(Inquire)
One-step extraction-free nucleic acid release agent, suitable for rapid lysis of RNA templates from serum, plasma, bronchoalveolar lavage fluid, and swab samples, without heating or nucleic acid extraction/purification process. Add this reagent to the sample when using and can extract RNA at room temperature in 10 min.
Features: Single solution, easy to operate, efficient, and fast. Compatible with various follow-up experiments, including qPCR, RT-qPCR, etc. The reagent is safe, toxic-free, environmentally friendly, and healthy. The product can be shipped and stored at room temperature. It can be used as a sample preservative to delay RNA degradation.
2.2 Hieff Unicon™ Fast Multiplex One Step RT-qPCR Probe Kit(UDG plus) (Cat#13660ES)(Inquire)
Designed for virus samples, virus detection can be quickly completed with only one tube of the reaction solution. This one-step method is suitable for fast cycling reactions, and experimental results can be obtained within 30 min.
Features: 30 min rapid amplification, suitable for rapid amplification procedures of various instruments such as Q5 and SLAN. Good reverse transcription specificity and extension performance. High amplification efficiency, high amplification sensitivity, and high amplification specificity. With dUTP/UDG anti-pollution system.
2.3 Case show
10 μL of pseudovirus RNA was obtained by using nucleic acid release agents from Yeasen and S* respectively. After the samples were stored for 4 hours, they were amplified and verified with RT-qPCR reagents. The results show that the nucleic acid obtained by Yeasen nucleic acid release agent has better sensitivity and amplification linearity when matched with a one-step RT-qPCR reagent.
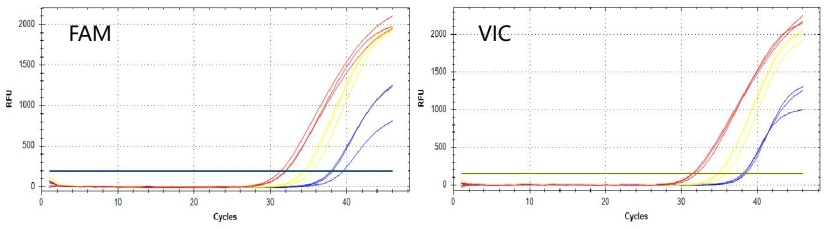
Figure 1. Extraction-free pseudovirus amplification curve
Note: Amplification Instrument and Procedure: Q5 Fast. Yellow: water; Blue: s*; Red: Yeasen
The virus sample storage time test of Yeasen nucleic acid release agent was verified by amplification with the RT-qPCR reagent. It was found that the amplification effect was still good after the samples were placed for 34 days.
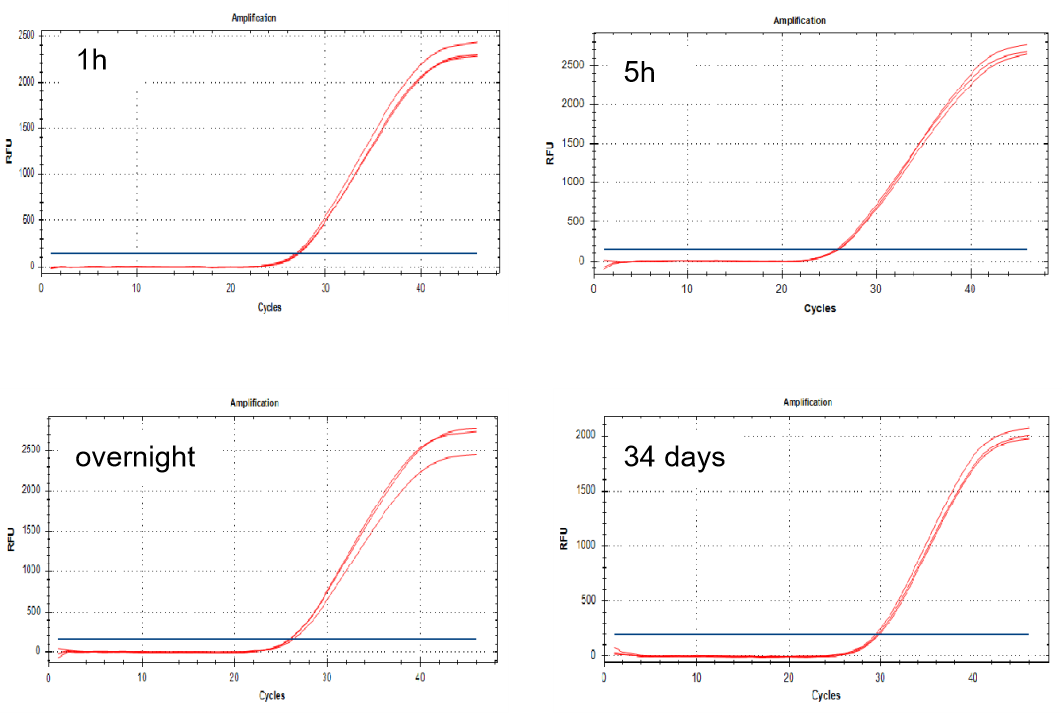
Figure 2. Virus RT-qPCR amplification curve under different treatment times of release agent
Note: Amplification Instrument and Procedure: Q5 Fast
3. What are the ways to reduce the cost of nucleic acid testing?
Traditional molecular diagnostic reagents have problems in shipping and storage. To ensure the biological activity of the active ingredients (DNase, reverse transcriptase, etc.) in the reagent, it is generally necessary to store and transport in a cold chain environment of around -20°C. Cold chain transportation requires a lot of ice packs or dry ice, which is costly, and changes in ambient temperature can easily cause repeated freezing and thawing of detection reagents, which will directly affect the performance and shelf life of the reagents. Lyophilization of liquid reagents into solid reagents will solve the above problems.

Figure 3: Lyophilized reagents
Vacuum freeze-drying, referred to as freeze-drying, refers to the process in which solid materials are directly sublimated to a gaseous state for drying under vacuum conditions. In the process of freeze-drying, the solvent is directly sublimated from a solid state to a gaseous state, so the original chemical structure and morphology of the solute will not be destroyed. The biologically active material can still restore the conformation and biological function before lyophilization after redissolving. Therefore, lyophilization is a drying method with minimal effect on the properties of the dried substance.
Freeze-drying technology has the following advantages: Freeze-drying is carried out at a low temperature, so it is especially suitable for many heat-sensitive substances. Such as proteins, microorganisms, and the like will not denature or lose biological activity. Therefore, it is widely used in medicine. When drying at low temperatures, the loss of some volatile components in the substance is very small, which is suitable for drying some chemical products, medicines, and food. During the freeze-drying process, the growth of microorganisms and the action of enzymes cannot proceed, so the original properties can be maintained. Since it is dried in a frozen state, the volume is almost unchanged, the original structure is maintained, and no concentration phenomenon occurs. The dried material is loose and porous, in the form of a sponge, dissolves rapidly and completely after adding water, and restores its original shape almost immediately. Since drying takes place under a vacuum with very little oxygen, some oxidizable substances are protected. Drying can remove more than 95-99% of the moisture so that the dried product can be stored for a long time without deterioration.
4. Product matching to reduce the cost of nucleic acid testing
Product collocation: Hieff Unicon™ V Lyo-nCoV Multiplex One Step RT-qPCR Kit(with MgCl2) (CAT#13775), qPCR Lyoprotectant (CAT#13743).
4.1 Hieff Unicon™ V Lyo-nCoV Multiplex One Step RT-qPCR Kit(with MgCl2) (CAT#13775)
A glycerol-free, ready-to-use molecular diagnostic reagent, containing thermostable reverse transcriptase, hot-start Taq DNA polymerase, reaction buffer, dNTPs, magnesium chloride, and lyoprotectant. The reagent is also added factors effectively inhibiting non-specific PCR amplification and factors improving the amplification efficiency of multiplex qPCR reactions. This product is an ideal choice for the development of multiplex RT-qPCR that has room temperature stability and can be shipped and stored at room temperature.
Advantages: After lyophilization, the enzyme activity remains intact, and the performance is the same as that of liquid reagents. After lyophilization, the product remains stable at room temperature and does not require cold chain transportation and low-temperature storage. Reduce cost and operational complexity: Provide reference freeze-drying process to help lyophilization process; no additional freeze-drying protective agent is required, and it can be used directly for lyophilization.
4.2 Partial data display
Some data of Hieff Unicon™ V Lyo-nCoV Multiplex One Step RT-qPCR Kit (with MgCl2) are as follows.
Table 1. Product data
|
Product data |
Specific |
|
Instrument |
7500 |
|
System |
25μL |
|
Template |
pseudovirus |
|
Template concentration |
105,104,103 copies/mL |
|
Procedure |
50°C 10 min, 95°C 5min, 45 cycles(95°C 15 sec, 60°C 15 sec) |
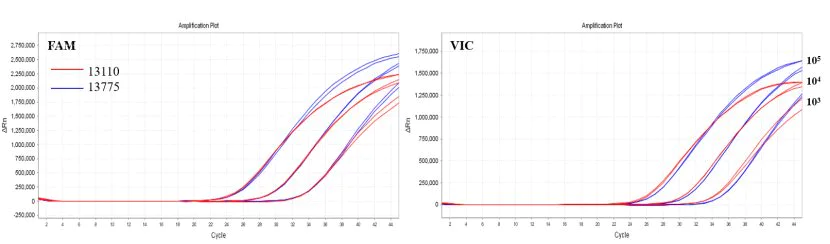
Figure 4. Liquid performance of 13775
Note: Multiplex RT-qPCR amplification using high-sensitivity RT-qPCR reagents 13110 (red) and 13775 (blue) for pseudovirus templates. The left graph is the FAM channel, the right is the VIC channel.
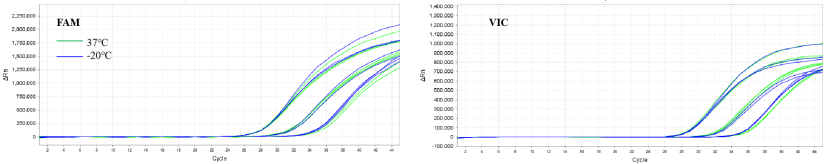
Figure 5. Thermal stability of 13775 liquid reagent at 37°C for 5 days
Note: The 13775 liquid reagent was placed at 37°C (green) and -20°C (blue) for 5 days for a multiplex RT-qPCR amplification test. The left graph is the FAM channel, the right is the VIC channel.
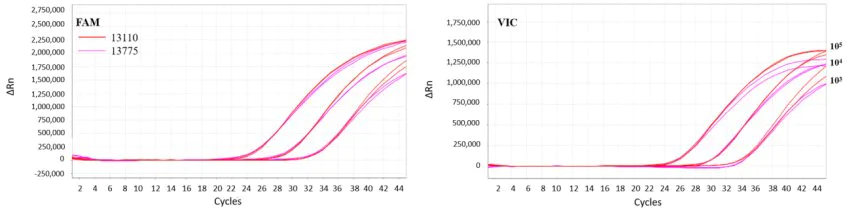
Figure 6. The performance of 13775 product after lyophilization
Note: The pseudovirus template was amplified by multiple RT-qPCR using liquid reagents (red) and 13775 lyophilized powder (purple) respectively. The left graph is the FAM channel, the right is the VIC channel. The results showed that the activity of the 13775 reagent was intact after lyophilization, and it still had high-efficiency multiple reaction ability.
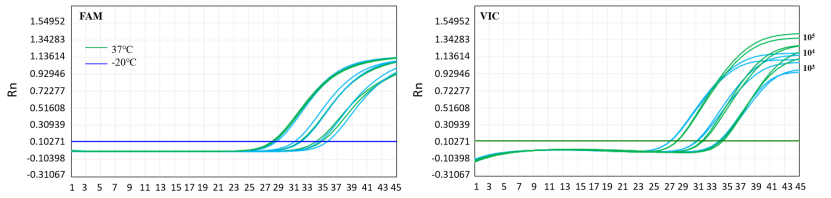
Figure 7. Thermal stability of 13775 lyophilized powder—37°C for 21 Days
Note: The 13775 lyophilized powder was placed at 37°C (green) and -20°C (blue) for 21 days for multiple RT-qPCR amplification tests. The left graph is the FAM channel, the right is the VIC channel. The results showed that the lyophilized powder still had a good amplification effect after being placed at 37°C for 21 days.
Table 2: Products provided by Yeasen
|
Product category |
Product name |
Cat# |
|
One-step fast RT-qPCR |
Hieff Unicon™ Fast Multiplex One Step RT-qPCR Probe Kit (UDG plus)(Inquire) |
13660ES |
|
Nucleic Acid Release Reagent(Inquire) |
13720ES |
|
|
Lyophilizable RT-qPCR |
Hieff Unicon™ V Lyo-nCoV Multiplex One Step RT-qPCR Kit (with MgCl2) |
13775ES |
|
13743ES |
||
|
conventional RT-qPCR |
13747ES |
|
|
13173ES |
The related products that Yeasen can provide are as follows:
Table 3: Related Products
|
Product category |
Product name |
Cat# |
|
Hot-start Taq DNA polymerase |
10717ES |
|
|
10723ES |
||
|
Monoclonal double-blocking anti-Taq DNA polymerase antibody |
31303ES |
|
|
Reverse Transcriptase |
11300ES |
|
|
11301ES |
||
|
Thermosensitive UDG |
10303ES |
Regarding reading:
How to choose drying reagents, Air Drying or Lyophilization?
Yeasen Biology's Overall Solution for African Swine Fever Virus Detection
African Swine Fever Virus - Total Master Mix/Direct Amplification qPCR Solution