D-Luciferin Firefly, Free Acid
Product Description
D-luciferin is a common substrate for Luciferase and is widely used throughout biotechnology, especially in vivo imaging Art. The mechanism of action is that luciferin (substrate) can be oxidized to emit light in the presence of ATP and luciferase. When fluorescein is in excess, the number of light quanta produced is similar to fluorescence The concentration of phototinase was positively correlated (see figure below). The plasmids carrying luciferase encoding gene (Luc) were transfected into cells and introduced into study animals such as mice and rats.
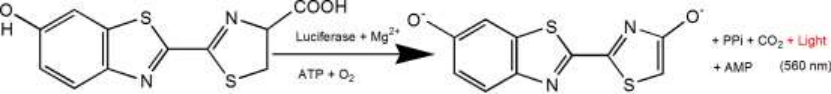
D-luciferin is also commonly used in in vitro studies, including luciferase and ATP levels analysis, reporter gene analysis, high-throughput sequencing, and various contamination detection.The current market It is available in three product forms, D-luciferin (free acid), D-luciferin sodium salt, and D-luciferin potassium salt. The main difference between these three products lies in the solubility properties. D-fluorescein (free acid) is water soluble and the solubility of buffering systems is weak, except in weak bases such as NaOH and KOH solutions. Sodium and potassium forms of D-fluorite Light element can be easily and quickly dissolved into water or buffer, convenient to use, the solvent is not toxic, especially suitable for in vivo experiments. These three products, when they're made into liquid, There is no substantial difference in the vast majority of applications.
Product Properties
|
English synonym |
(S)-4,5-Dihydro-2-(6-hydroxy-2-benzothiazolyl)-4-thiazolecarboxylic acid; D-Luciferin Firefly, free acid |
|
CAS NO. |
2591-17-5 |
|
Formula |
C11H8N2O3S2 |
|
Molecular weight |
280.33 g/mol |
|
Appearance |
Light yellow powder |
|
Solubility |
This product is difficult to dissolve in water, but dilute alkali can be added to promote its dissolution. |
|
Purity (HPLC) |
≥95% |
Shipping and Storage
The products are shipped with ice pack and can be stored at -20℃ to -80℃ for 1 year.
Instructions
1. In vitro bioluminescence detection
1) Dilute alkali (such as NaOH, KOH solution) was used to dissolve D-luciferin and free acid to prepare 30 mg/mL storage solution (200×) and adjust the pH to 7.4. After mixing, use immediately or store separately at -20℃ or -80℃ to avoid repeated freeze-thaw.
[Note]: If precipitation occurs, the pH needs to be adjusted to a higher level until complete dissolution. It can then be neutralized again with an acidic solution and adjusted to pH7.4.
2) The preheated tissue culture medium was used to dilute the storage solution at 1:200 to prepare the working solution (final concentration 150 µg/mL).
3) Cell culture medium was removed.
4) Before image analysis, fluorescein working solution was added into the cells and incubated at 37℃ for few time, then image analysis was performed.
2. In vivo imaging analysis
1) D-fluorescein working solution (15 mg/mL) was prepared with dilute alkali (such as NaOH, KOH solution), and the pH was adjusted to 7.4, 0.2 µm filter membrane for sterilization. After blending Use immediately or store separately at -20℃ or -80℃ to avoid repeated freezing and thawing. Once in use, defrost at 4 ℃, keep cold and out of light.
2) The amount of injection depends on the method of injection, as follows:
|
Injection method |
Dose |
|
Iv (25-27gauge needle) |
Add a corresponding volume of 15 mg/mL fluorescein working solution at the concentration of 10 µL/g body weight |
|
intraperitoneal injection |
Add a corresponding volume of 15 mg/mL fluorescein working solution at the concentration of 10 µL/g body weight |
|
intramuscular injection |
50 µL, the concentration of 1-2 mg/mL fluorescein working solution |
|
nasal injection |
50 µL, the concentration of 1-2 mg/mL fluorescein working solution |
2) Imaging analysis was performed after 10-20 min of injection (when the optical signal reached the maximum stable plateau).
[Note]: It is suggested to establish a luciferase kinetic curve for each animal model, so as to determine the maximum signal detection time and signal plateau period.
Matters needing attention
1) Firefly Luciferin and Beetle Luciferin both refer to the compound (S)-2-(6-hydroxy-2-benzothiazolyl)-2-thiazoline-4- Carboxylic acid, just the name difference between different companies.
2) Avoid light during storage and operation. In addition, after the storage solution is filtered and sterilized, it can be frozen separately at -20℃ or -80℃ to avoid repeated freezing and thawing. If I can The storage solution is filled with nitrogen or argon (to prevent oxidation) for longer stability and storage time of up to 1 year.
3)The injection method, animal type, and body weight will all affect the signal emission, so it is recommended that a luciferase kinetic curve be done for each experiment to determine the optimal signal level Stage time and the best detection time
4) For your safety and health, please wear a lab coat and disposable gloves to operate.
Cautions
1. The product should be used with YEASEN high resolution gradient precast adhesive (Cat# 36245-Cat# 36256), not recommended with another precast adhesive.
2. This buffer contains no SDS and is used for natural protein electrophoresis.
3. For your safety and health, please wear a lab coat and disposable gloves to operate.
4. This product is for research use ONLY!
[1] Ji C, Zhao M, Wang C, et al. Biocompatible Tantalum Nanoparticles as Radiosensitizers for Enhancing Therapy Efficacy in Primary Tumor and Metastatic Sentinel Lymph Nodes [published online ahead of print, 2022 Jun 6]. ACS Nano. 2022;10.1021/acsnano.2c02314. doi:10.1021/acsnano.2c02314(IF:15.881)
[2] Miao Z, Tian W, Ye Y, et al. Hsp90 induces Acsl4-dependent glioma ferroptosis via dephosphorylating Ser637 at Drp1. Cell Death Dis. 2022;13(6):548. Published 2022 Jun 13. doi:10.1038/s41419-022-04997-1(IF:8.469)
[3] Zhang L, Sun Y, Zhang XX, et al. Comparison of CD146 +/- mesenchymal stem cells in improving premature ovarian failure. Stem Cell Res Ther. 2022;13(1):267. Published 2022 Jun 21. doi:10.1186/s13287-022-02916-x(IF:6.832)
[4] Fu RJ, He W, Wang XB, et al. DNMT1-maintained hypermethylation of Krüppel-like factor 5 involves in the progression of clear cell renal cell carcinoma. Cell Death Dis. 2017;8(7):e2952. Published 2017 Jul 27. doi:10.1038/cddis.2017.323(IF:5.965)
[5] Xu P, Wu L, Cao M, et al. Identification of MBW Complex Components Implicated in the Biosynthesis of Flavonoids in Woodland Strawberry. Front Plant Sci. 2021;12:774943. Published 2021 Nov 8. doi:10.3389/fpls.2021.774943(IF:5.754)
[6] Bai S, Tao R, Yin L, et al. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019;100(6):1208-1223. doi:10.1111/tpj.14510(IF:5.726)
[7] Yang SX, Wu TT, Ding CH, Zhou PC, Chen ZZ, Gou JY. SAHH and SAMS form a methyl donor complex with CCoAOMT7 for methylation of phenolic compounds. Biochem Biophys Res Commun. 2019;520(1):122-127. doi:10.1016/j.bbrc.2019.09.101(IF:2.705)
Catalog No.:*
Name*
phone Number:*
Lot:*
Email*
Country:*
Company/Institute:*
Recommended products

