YEASEN UCF. ME ® UltraNuclease(Benzonase Nuclease) —— Your BEST choice for degrading nucleic acids
UCF.ME™ UltraNuclease is also called Totipotent nuclease, broad-spectrum nuclease, and non-limiting Inhibitory endonuclease. It is originally derived from Serratia Marcescen, then it was reorganized and constructed by Yeasen Research & Development team and expressed in E. coli. The molecular weight is 26.5 kD and the isoelectric point is 6.85. Surprisingly, it can completely digest Nucleic acid into 5'-monophosphate oligonucleotides with a length of 2-5 bases. What are the application scenarios of totipotent nuclease, and what properties should a good totipotent nuclease possess?

Figure 1. UCF.ME™ UltraNuclease
1. What is totipotent nuclease?
2. What are the usage scenarios of totipotent nuclease?
3. What are the advantages that totipotent nuclease needs to have?
4. FAQ
5. Related Products
1. What is totipotent nuclease?
Totipotent nucleases are genetically engineered endonucleases that degrade all forms of DNA and RNA, including single-stranded, double-stranded, linear, native, and denatured nucleic acids. And it generates 5-monophosphate oligonucleotides with a length of 3 to 5 bases and does not have base recognition specificity. Totipotent nucleases can have high stability and digestion activity under various conditions, thereby efficiently removing nucleic acid residues in samples or products, improving the purity of samples and the biological efficacy of products, and can be used in a variety of scientific research and vaccines, protein, polysaccharide pharmaceutical industry's preferred enzyme preparation. It is also the removal of nucleic acid during the preparation of vaccines, antibodies, cell therapy, and other biological products so that it can meet the FDA's requirement that the residual nucleic acid in the final product of biological preparations for therapeutic use should not exceed 10pg/dose. Totipotent nucleases can reduce the viscosity of cell lysate by degrading nucleic acid, which helps to promote the solution filtration/ultrafiltration process in the purification process of cell-derived particles such as viruses, AAV vectors, and inclusion bodies, shorten processing time and improve centrifugation. Improve the separation efficiency of precipitation and supernatant, improve the efficiency of chromatographic purification, and achieve the effect of improving the yield and product purity.
2. What are the usage scenarios of totipotent nuclease?
UltraNuclease has a wide range of applications and here are the specific usage scenarios.
2.1 Virus purification (lentivirus, AAV, recombinant adenovirus vaccine, inactivated virus, oncolytic virus, etc.)
The use of viruses in the biomedical industry is becoming more and more extensive, including vaccines, cell therapy, gene therapy, and other fields. The host cells that produce viruses are mostly continuous passage cells (CCLs). CCLs have a genetic failure to regulate growth and have the ability to proliferate indefinitely. The residual DNA of CCLs may make human cells proliferate out of control and become tumor cells. In addition, there may be infectious viral genomes in the residual DNA, and the risk of infection may be higher than the risk of tumorigenesis. Therefore, companies need to strictly control the residual DNA of biological products to ensure their safety of biological products. That is to say, you can apply to our UCF.ME™ UltraNuclease to purify the virus securely.
2.2 Purification of the recombinant protein expressed in E. coli
For some proteins expressed by E. coli, it is easy to form inclusion bodies, which are often entangled with the host DNA during the purification process. The broken bacterial cells are often very vicious, which greatly reduces the purification efficiency of the protein. When ultrasonically disrupting the bacteria, if you add 10-50 U/mL UCF.ME™UltraNuclease to the bacteria solution, and incubate the sample at 37°C for 30 min, or 4°C overnight after the disruption is complete, it can effectively reduce the viscosity of the bacterial liquid and greatly improve the purification efficiency. In a word, you can rely on our UCF.ME™ UltraNuclease to purify the protein effectively.
2.3 Prevent cell clumping
In recent years, the use of cryopreserved peripheral blood mononuclear cells (PBMC) in immunoassays has increased dramatically. The biggest characteristic of PBMC cells is that they are very easy to clump after resuscitation, which can easily lead to low cell quality and unreliable test results. When the cells are resuscitated, the appropriate concentration (25-50 U/mL) of UCF.ME™UltraNuclease is added to the culture medium and the cells are co-cultured, which can effectively prevent cell clumping. Hence, you can make the most of our UCF.ME™ UltraNuclease to increase the odds of a successful outcome.
3. What are the advantages that totipotent nuclease needs to have?
3.1 Wide range of applications
UCF.ME™ UltraNuclease can cut and degrade various forms of DNA and RNA under a variety of experimental conditions and can be applied to remove residual nucleic acid in various biological products.
3.2 High purity, high enzyme activity
For our UCF.ME™ UltraNuclease, its enzyme purity is greater or equal to 99% (HPLC), and its enzyme activity is greater or equal to1.5*106 U/mg (using an absolute quantitative method for general substrate).
3.3 Strong adaptability
UCF.ME™ UltraNuclease has high stability in storage and freeze-thaw. We store UCF.ME™ UltraNuclease at -20 °C, 4 °C, and 37 °C to detect the trend of enzyme activity. When placed at 37 °C for 2 weeks, there was no significant change in enzyme activity. Meanwhile, to ensure the stability of the enzyme, we use a combination of dry ice + ice pack to transport UCF.ME™ UltraNuclease to your place. It also has strong tolerance and can adapt to a variety of operating conditions.
3.4 Comply with Pharmacopoeia
UCF.ME™ UltraNuclease is manufactured in UCF.ME (Ultra Clean Factory. Molecular Enzyme) is a kind of Ultra-clean enzyme. What is an Ultra-clean enzyme? It is a molecular enzyme that achieves quality standards of no HCD、HCR、HCP, DNASE FREE、RNASE FREE, no protease residues, no microbial residues, no pathogens, animal origin, antibiotic pollution, ultra-low endotoxin Residual, high enzyme activity, and high purity, etc. Hence, you can trust our products, safely and reliably.
3.5 GMP production
The whole process of UltraNuclease production is completed in the GMP production workshop, and the quality inspection plan is strictly by the pharmacopeia method, meeting the large-scale demand from research and development to production.
UCF.ME™ UltraNuclease’s partner —— UltraNuclease ELISA kit
In normal purification steps, UCF.ME™ UltraNuclease is easily removed as an impurity. To detect the residue of UCF.ME™ UltraNuclease, YEASEN has developed the supporting UltraNuclease ELISA kit, which can accurately detect the residue of UCF.ME™ UltraNuclease in the sample. The kit has excellent linearity, repeatability, recovery rate, and specificity.
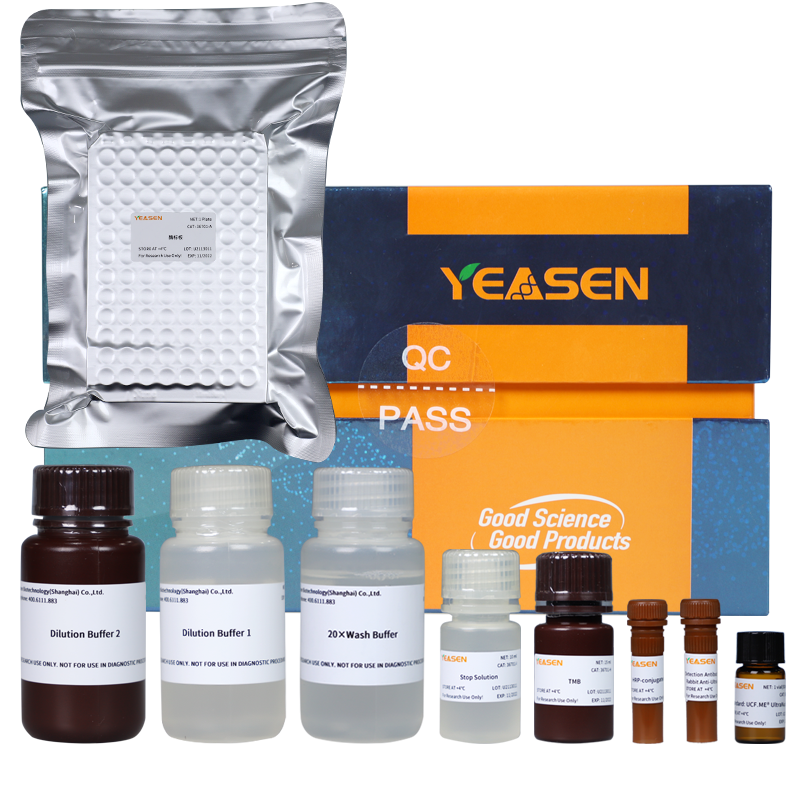
Figure 2. UltraNuclease ELISA kit
The linear range is between 0.047 and 3 ng/mL, and R2 is greater than 0.99. with Good inter-batch reproducibility, we add a fixed concentration of UltraNuclease to the sample and use 3 different batches of ELISA kits for detection. The detection recovery rate is between 70% and 110% when the nuclease concentration is between 3 and 0.186 ng/mL, and CV is less than 10%. What’s more, the simulated sample recovery rates of UltraNuclease with different concentrations are all between 70% and 130% and are better than other brands. It also has strong specificity that we evaluated the non-specific binding of the UltraNuclease antibody to 8 common samples. The results of the interference group and the control group overlapped, and no interference was seen.
4. FAQ
Q1: In which step to add UltraNuclease?
A: It depends on the samples you are dealing with. In virus purification, it is generally added after virus clarification; in E. coli protein purification, it can be added when the cells are lysed; in dealing with cell clumping, it can be directly added to the medium for co-cultivation with cells.
Q2: If the reaction temperature cannot reach 37°C, how to use it?
A: The enzyme activity of UltraNuclease is affected by temperature, treatment time, and enzyme activity units. If the temperature cannot reach 37°C, the amount of enzyme can be added appropriately, or the incubation time can be extended.
Q3: Is UltraNuclease compatible with protease inhibitors?
A: Compatible, but be aware that many protease inhibitors contain EDTA. When EDTA starts to be higher than 1mM, EDTA will inhibit the activity of some nucleases.
Q4: How to remove UltraNuclease?
A: It can be removed by methods such as TFF, dialysis, and chromatographic columns.
Q5: Can the UltraNuclease ELISA kit be used for the detection of other brands of totipotent nuclease residues?
A: We do not recommend doing this. The ELISA kit is completely developed based on UltraNuclease and can accurately quantify UltraNuclease. Altipotent nucleases of other brands may be different from UltraNuclease in terms of sequence and production process, and the test results may be inaccurate.
5. Related Products
The products provided by Yeasen are as follows.
Table 1 Related Products
| Product name | SKU | Specifications |
| UCF.ME™ UltraNuclease GMP-grade | 20157ES25/60/80/90 | 25KU/100KU/1MU/5MU |
| UltraNuclease ELISA kit | 36701ES59 | 96T |