Salt Active UltraNuclease GMP-grade
Product description
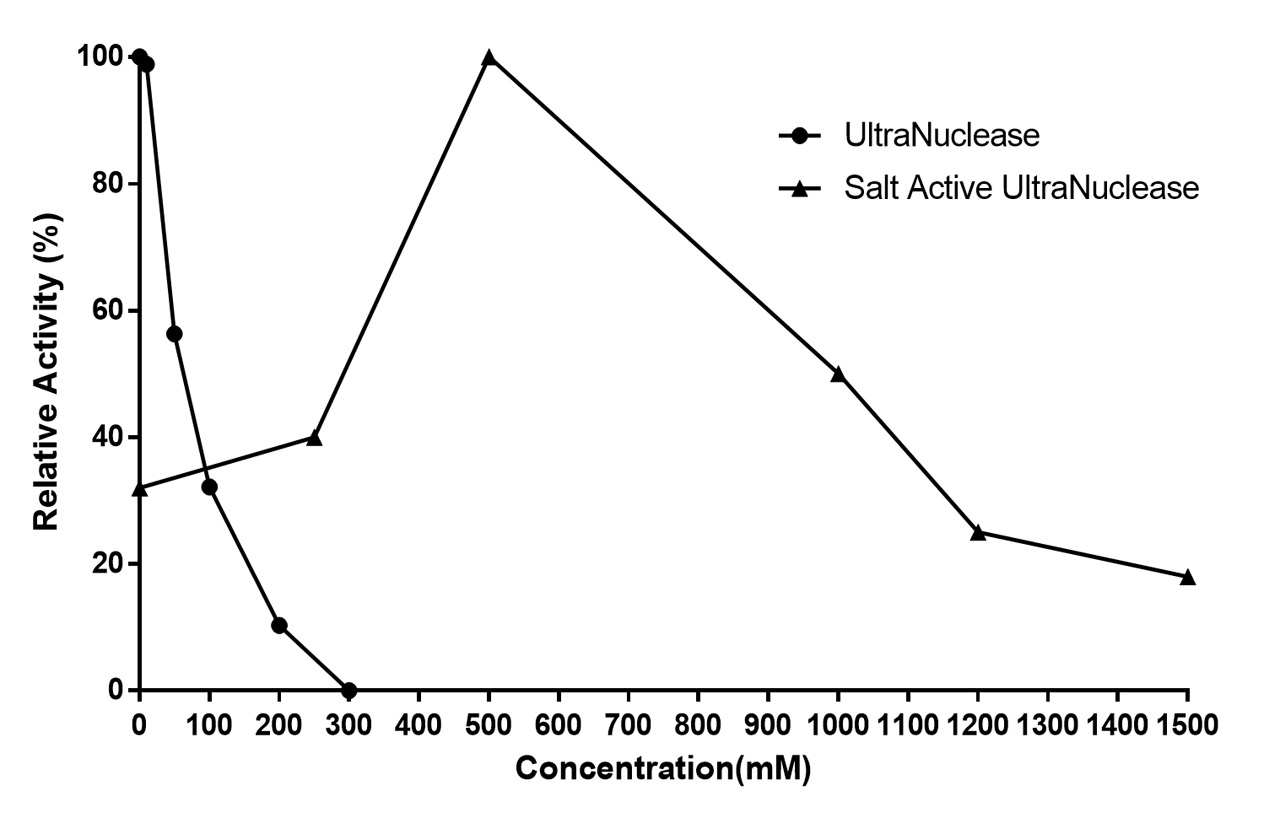
Salt Active UltraNuclease is a nonspecific endonuclease and has optimum activity at high salt concentration. Salt can play an important role in minimizing aggregation of protein or virus, which would be helpful for dissociation of DNA & RNA from proteins or other cellular components. In high salt conditions, enzymes are more likely to access the released DNA & RNA and degrade them. Nuclease which has high activity at high salt would be more effective to improve purification processes. The Salt Active UltraNuclease can be used to reduce the viscosity of cell supernatant and cell lysate, increase purification efficiency in high salt condition.
The enzyme can also reduce host nucleic acid residues to pg-grade, improving the performance and safety of biological products of applications including virus purification, vaccine manufacturing, and protein/polysaccharide pharmaceutical manufacturing.
This product is produced by GMP process requirements and provided in a liquid form with the following features.
- High purity (≥ 98%)
- Animal-free production
- Low Endotoxin
- High activity at high salt conditions
- Supported by ELISA Kit
Components
|
Components No. |
Name |
20159ES25 (25 KU) |
20159ES60 (100 KU) |
20159ES80 (1 MU) |
20159ES90 (5 MU) |
|
20159 |
Salt Active UltraNuclease GMP-grade (250 U/μL) |
100 μL |
400 μL |
4 mL |
20 mL |
Specification
|
Expression Host |
Recombinantly produced in Escherichia coli |
|
Molecular Weight |
24.7kDa |
|
Isoelectric point |
9.61 |
|
Purity |
≥ 99% |
|
Storage Buffer |
25 mM Tris-HCl,5 mM MgCl2,500 mM NaCl,50% glycerol |
|
Unit Definition |
The definition of one activity unit (U) is the amount of enzyme that causes a △A260=1.0 in 30 minutes at 37℃ in the excess of substrate at the certain condition. |
Shipping and Storage
The product should be stored at -25 ~ -15℃ for two years. If the product is opened and has been stored at 4℃ for more than one week, we recommend filtering the product to prevent microbial contamination.
Instructions
1. Sample Collection
Adherent cells: remove the medium, wash the cells with PBS, and remove the supernatant.
Suspension cells: collect the cells by centrifugation, wash the cells with PBS, centrifuge at 6,000 rpm for 10 min, collect the pellet.
Escherichia coli: collect the bacteria by centrifugation, wash once with PBS, centrifuge at 8,000 rpm for 5 min, and collect the pellet.
2. Sample Treatment
Treat the collected cell pellets with lysis buffer at the ratio of mass(g) to volume(mL) 1:(10-20), or by mechanical or chemical methods on ice or at room temperature (1g of cell pellet contains about 109 cells).
3. Enzyme Treatment
Add the moderate amount of MgCl2 to the reaction system and adjust the pH to 8-9.
Add the enzyme according to the ratio of 250 Units to digest 1 g of cell pellets, incubate at 37°C for more than 30 minutes. If you reduce the enzyme input or decrease the reaction temperature, you should extend the reaction time appropriately to get the equivalent digestion performance.
4. Supernatant Collection
Centrifuge at 12,000 rpm for 30 minutes and collect the supernatant.
Note: If the solution is acidic or alkaline, or contains high concentrations of salt, detergents, or denaturants, please increase the enzyme dosage or extend the treatment time accordingly.
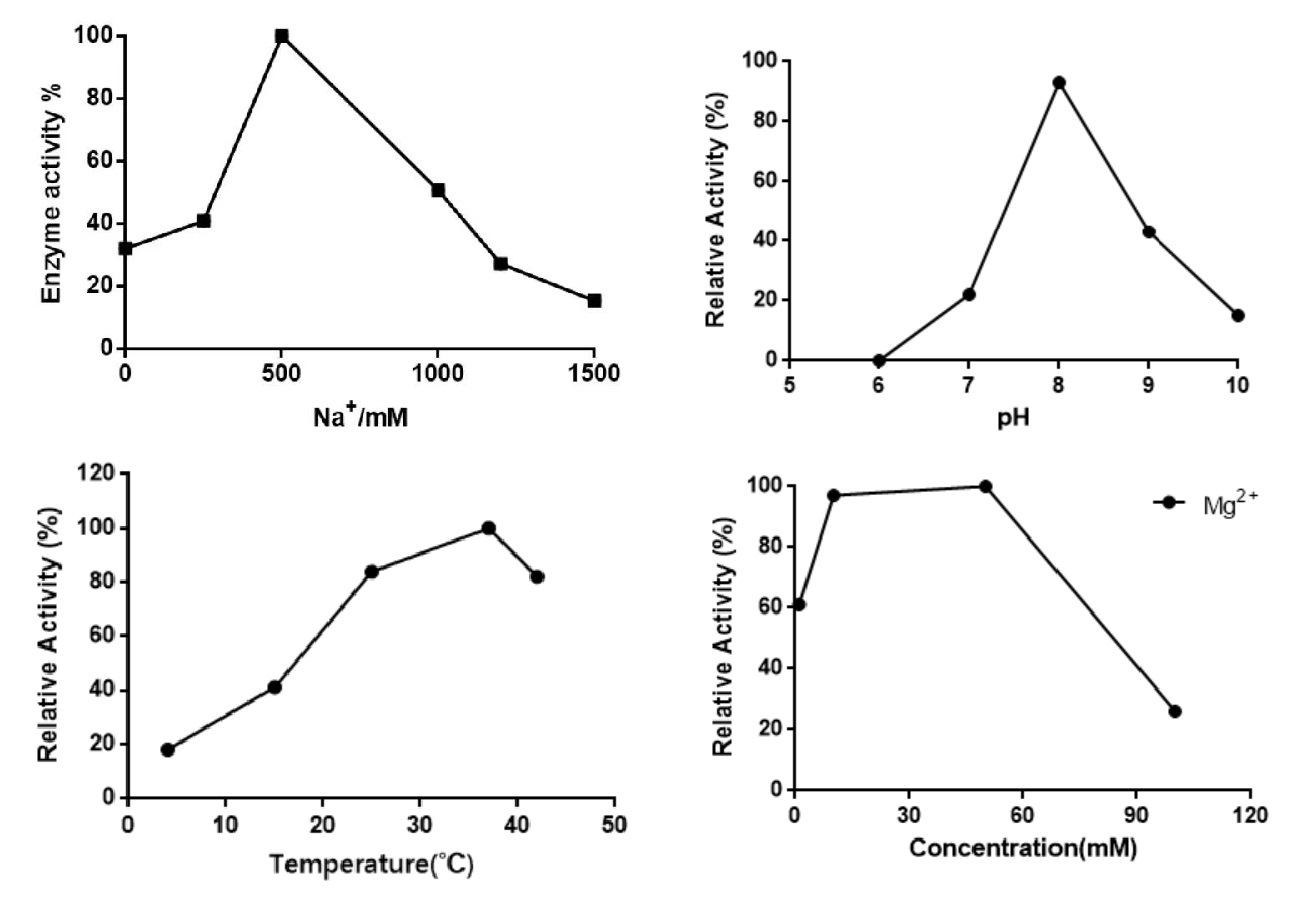
Recommended operating conditions
|
Condition |
Optimal |
Effective |
|
Mg2+/Mn2+ |
10~50mM |
1~100mM |
|
pH |
8 |
6.5 ~10 |
|
Temperature |
37℃ |
4~42℃ |
|
Na+/K+ |
500mM |
300~1200mM |
Recommended Usage
|
Condition |
Suggested usage |
|
Protein |
100U/mL |
|
Cell lysate |
500U/mL |
|
Cell extract |
1000U/mL |
|
Viscosity reduction |
50U/mL |
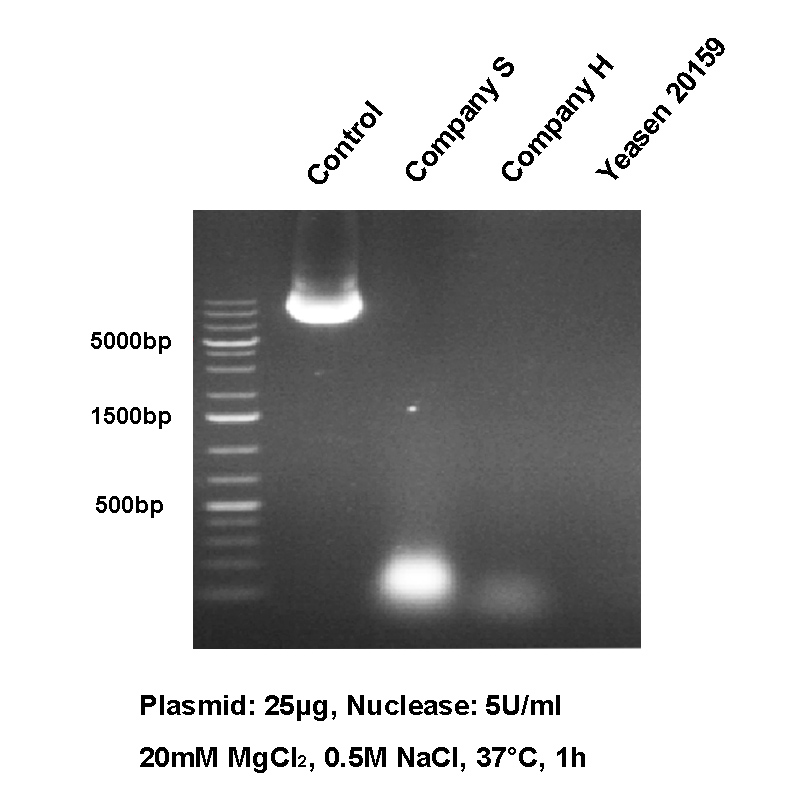
AAV Production Case
|
Supplier |
Condition (100U/mL, 37℃, 1h) |
HCD residuals(ng/ml) |
|
M* |
400mM NaCl |
1.42 |
|
M* |
400mM NaCl,1% Tween |
0.68 |
|
Yeasen 20159ES |
400mM NaCl |
0.87 |
|
Yeasen 20159ES |
400mM NaCl,1% Tween |
0.07 |
Documents:
Manuals
Notes
Please wear the necessary PPE, such lab coat and gloves, to ensure your health and safety!
Catalog No.:*
Name*
phone Number:*
Lot:*
Email*
Country:*
Company/Institute:*
Recommended products