When the terms "molecular cloning" or "vector creation" are spoken, the first thing that may come to mind is the classic enzyme ligation method, which involves the use of restriction enzymes to form sticky ends and T4 DNA ligase for the ligation of two fragments. Cloning technique. This approach has clear drawbacks, such as a lengthy ligation reaction time, laborious operation, limited cloning efficiency, and a restricted ability to choose enzyme cleavage sites.
To increase cloning efficiency and decrease reaction time, a homologous recombinase-based seamless cloning method was developed. Compared to the standard enzyme ligation technology, the seamless cloning technology is easy to use, quick, and not restricted by the enzyme cleavage site. It can simultaneously splice several fragments, which dramatically boosts cloning efficiency.
Yeasen Biotechnology obtained high-purity recombinase, continually refined the microenvironment of the recombinase reaction, and produced the Hieff Clone™ series of seamless cloning kits using the mature tool enzyme expression and purification platform.
The Hieff Clone™ Cloning Kit can efficiently clone 50 bp-10 kb segments and can clone PCR results into any location of any vector. Introduce 15-25 bp of homologous sequences at the 5' ends of the forward and reverse PCR primers of the insert at the ends of the linearized vector, such that the 5' and 3' ends of the PCR products of the insert are respectively with the linearized vector. There are two identical sequences that correspond to the two endings. Under the influence of the recombinase, the PCR product and the linearized vector can be employed for subsequent transformation in as little as 5 minutes, directed cloning is performed, and the cloning success rate can exceed 95%.
1. Product advantages
2. Experimentation
3. Product showcase
4. Feedback
5. FAQ
6. Some published articles
7. Ordering Information
1. Product advantages
● No need to consider the restriction site: Insert fragment restriction site-free, compatible with any vector, sticky or blunt ends.
● Easy-to-use: Insert a 20-bp vector-end homolog at the 5' end of the inserted fragment's PCR primer.
● Fast and efficient: Recombination takes 5 minutes at 50 °C. Clones can be 95% successful.
● Multipurpose: Single-fragment or multi-fragment directional cloning of 50 bp-10 kb pieces; can be used with Canace high-fidelity enzyme for site-directed mutagenesis.
2. Experimentation
① Preparation of linearized vector: preparation of linearized vector by enzyme digestion or PCR.
② Preparation of insert fragment: At the 5' end of the upstream and downstream primers of the target DNA segment, add 15-25 bp homologous sequences to the vector; PCR amplifies the target fragment.
③ Recombination reaction: React at 50°C for 5-20 min with linearized vector and target DNA fragment.
④ Transformation and plating: Positive clones are found by transforming and plating recombinant products.
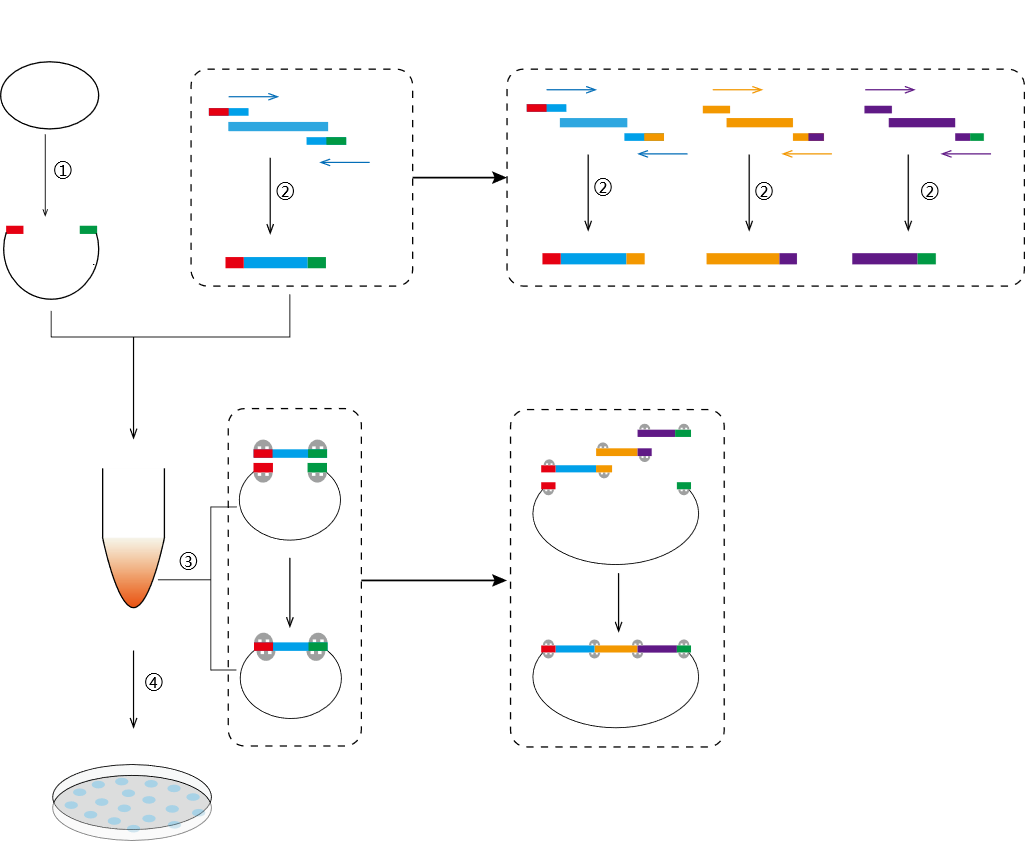
Figure 1. Single- and multi-fragment cloning with the Hieff Clone™ One-Step Directed Cloning Kit
3. Product showcase
3.1 Efficient single-fragment recombination
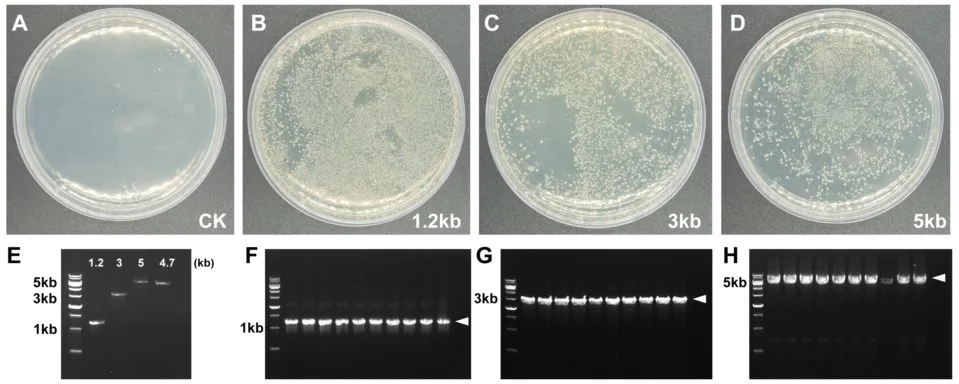
Figure 2. Single-fragment recombination
A-D: Recombinant transformation plates. E: Electropherogram of insert and vector concentration detection. F-H: Electropherogram of insert PCR identification. Arrows indicate target bands. Vector: pFastBac1, 4.7 kb, insert sizes are 1.2 kb, 3 kb and 5 kb, respectively, molar ratio of vector to insert: 1:3, recombination reaction conditions: 50 °C, 20 min.
3.2 Multi-segment recombination easily
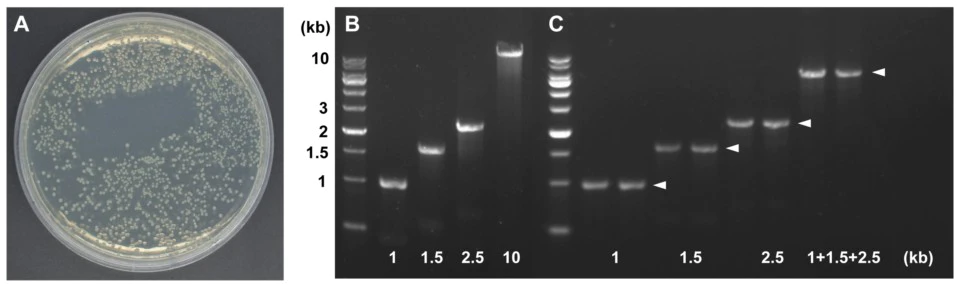
Figure 3. Multi-segment recombination
A: Recombinant transformation plate. B: Electropherogram of insert and vector concentration detection. C: PCR method to identify every single fragment and final linked gene. Arrows indicate target bands. Vector: pCAMIBA1302, 10 kb, three insert lengths: 1 kb, 1.5 kb and 2.5 kb, recombination reaction conditions: 50 °C, 20 min.
3.3 Efficient recombination of single fragment and five fragments
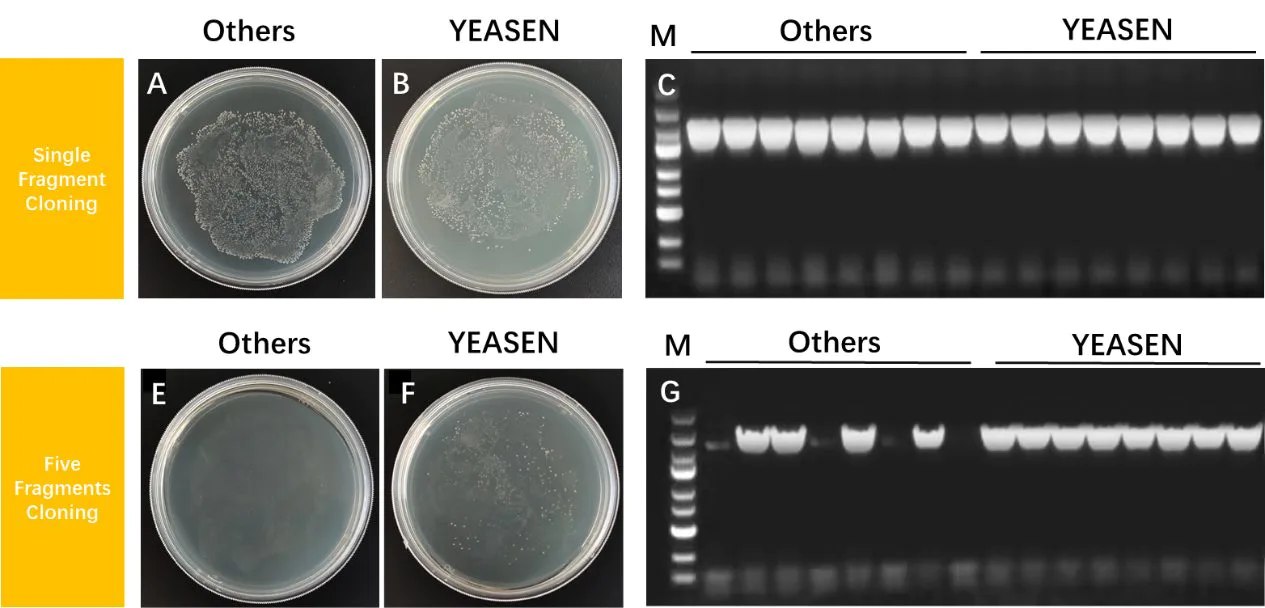
Figure 4. Recombination of a single fragment and five fragments
The Hieff Clone™ Universal One Step Cloning Kit ensures efficient single- and multi-fragment cloning. A-B: Recombinant transformation plates. C: Electropherogram of insert PCR identification. Vector: pGEX4T-1, 5 kb, insert length: 2 kb, molar ratio of 1:3, recombination reaction conditions: 50 °C, 5 min. E-F: Recombinant transformation plates. G: Electropherogram of insert PCR identification. Vector: pGEX4T-1, 5 kb, five inserts: 0.5 kb, 0.5 kb, 0.5 kb, 0.5 kb and 1 kb, recombination reaction conditions: 50 °C, 30 min.
4. Feedback
4.1 Long fragment recombination
Experimental information: vector size: 8000 bp; target fragment size: 7500 bp. Experimental data:
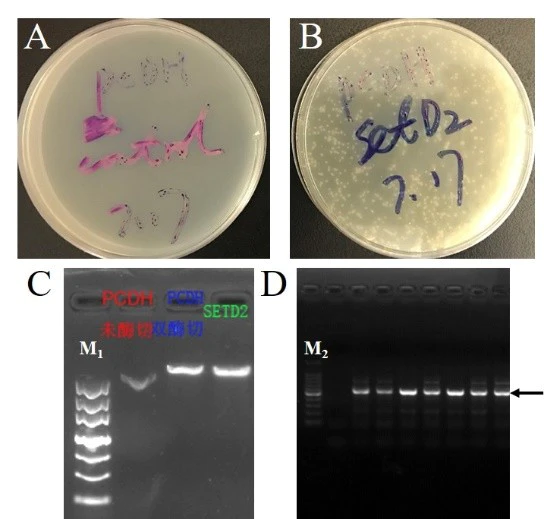
Figure 5. Long fragment recombination
A: Negative control transformation plate. B: Recombinant plasmid transformation plate. C: Electropherogram of concentration detection of vector and target fragment. D: Colony PCR identification electropherogram, arrows indicate target bands. M1: Marker III; M2: 100 bp DNA ladder. (Yan Chai Hospital)
4.2 Multi-fragment recombination
Experimental information: vector size: 3996 bp; four fragment sizes: 576 bp, 867 bp, 915 bp, and 647 bp. Experimental data:
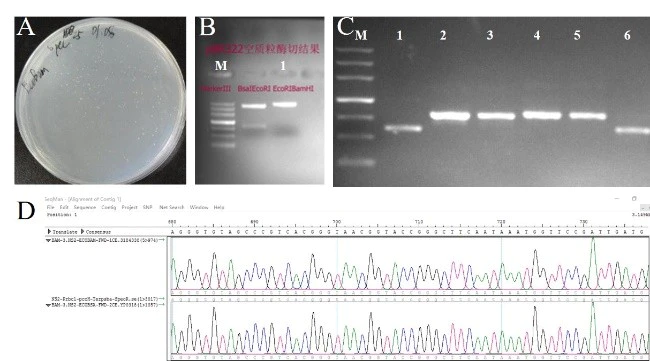
Figure 6. Multi-fragment recombination
A: Recombinant plasmid transformation plate. B: 1 is the agarose gel electrophoresis image after vector digestion. C: Colony PCR verification results, of which 1 is the original plasmid control electrophoresis, 2-5 are recombinant plasmids, only a target fragment of 863 bp is detected, and 6 is a false positive. D: Sequencing results of positive clones. M: Marker II. (Institute of Microbiology, Chinese Academy of Sciences)
5. FAQ
Q: What is the principle of Hieff Clone™ cloning?
A: Using the principle of terminal homologous recombination. Any linearized vector and DNA fragments with about 20 bp homologous sequences at both ends of the vector are rapidly and directionally cloned.
Q: What are the advantages of Hieff Clone™ clones compared to traditional clones?
A: 1) No need to consider the restriction site: it is not restricted by the restriction site of the insert fragment, applicable to any vector; the insert fragment is compatible with sticky or blunt ends.
2) Simple design: introduce a sequence of about 20 bp homologous to the end of the vector at the 5' end of the PCR amplification primer of the insert.
3) Fast and efficient: the recombination reaction can be completed in 20 minutes at 50°C. The positive rate of clones can reach more than 95%.
4) Wide range of applications: It can be used for single- or multi-fragment directional cloning, and can also be combined with Canace high-fidelity enzyme for site-directed mutagenesis.
Q: How does the length of the homologous sequence affect the recombination efficiency?
A: The length of the homologous sequence is related to the number of clones. Generally, the homologous fragment is selected at about 20 bp and can be adjusted within the range of 15 bp-25 bp. And choose to avoid secondary structures as much as possible.
Q: Will the quality of the linearized vector and target fragment affect the recombination reaction?
A: The poor quality of the linearized vector or the target fragment will greatly affect the recombination reaction. It is recommended to recover the purified product by tapping gel.
Q: The amount and ratio of the linearized vector and the amplification product of the target fragment?
A: The amount of vector used should be greater than 0.01 pmol, and the recommended amount should be 0.03 pmol; the molar ratio of cloning vector to target fragment should be in the range of 2:1-1:5, and the most suitable one is 1:2-1:3. affect cloning efficiency.
Q: What are the applicable experiments of the one-step rapid directional cloning kit?
A: This kit is suitable for the vast majority of cloning experiments based on the conventional "enzyme cleavage-ligation" method, and is especially suitable for multi-point mutation of genes and total gene synthesis that are difficult to achieve quickly by other conventional methods.
Q: Can the multi-fragment one-step rapid directional cloning kit be used for single-fragment cloning?
A: The multi-fragment one-step cloning kit can be used for single-fragment cloning. However, the single-fragment cloning kit is not recommended for multi-fragment cloning, and the recombination efficiency may be affected.
Q: The reaction temperature of the one-step rapid directional cloning kit is 50°C. Can the reaction be performed at 37°C?
A: In general, it is recommended to carry out the reaction at 50°C. 50°C is beneficial to eliminate the secondary structure of DNA and is the optimum reaction temperature for Hieff Clone™ recombinase. Recombination reaction can also be carried out at 37°C, please optimize it according to the experimental needs.
6. Some published articles
[1]. Jiang Y, Wang W, Xie Q, et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017. (IF47.728)
[2]. Liu CX, Li X, Nan F, et al. Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity. Cell. 2019. (IF41.582)
[3]. Xing YH, Yao RW, Zhang Y, et al. SLERT Regulates DDX21 Rings Associated with Pol I Transcription. Cell. 2017. (IF41.582)
[4]. Wang Y, Hu SB, Wang MR, et al. Genome-wide screening of NEAT1 regulators reveals cross-regulation between paraspeckles and mitochondria. Nat Cell Biol. 2018. (IF28.824)
[5]. Li X, Liu CX, Xue W, et al. Coordinated circRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Mol Cell. 2017. (IF17.970)
[6]. Yao RW, Xu G, Wang Y, et al. Nascent Pre-rRNA Sorting via Phase Separation Drives the Assembly of Dense Fibrillar Components in the Human Nucleolus. Mol Cell. 2019. (IF17.970)
[7]. Wu P, Zhang T, Liu B, et al. Mechano-regulation of Peptide-MHC Class I Conformations Determines TCR Antigen Recognition. Mol Cell. 2019. (IF1 17.970)
[8]. Qiao HH, Wang F, Xu RG, et al. An efficient and multiple target transgenic RNAi technique with low toxicity in Drosophila. Nat Commun. 2018. (IF14.919)
[9]. Liu C, Zheng S, Gui J, et al. Shortened Basal Internodes Encodes a Gibberellin 2-Oxidase and Contributes to Lodging Resistance in Rice. Mol Plant. 2018. (IF13.164)
[10]. Du L, Dong S, Zhang X, et al. Selective oxidation of aliphatic C-H bonds in alkylphenols by a chemomimetic biocatalytic system. Proc Natl Acad Sci U S A. 2017. (IF11.205)
[11]. Wang M, Nie Y, Wu XL. Extracellular heme recycling and sharing across species by novel mycomembrane vesicles of a Gram-positive bacterium. ISME J. 2021. (IF10.302)
[12]. Ma F, Yang X, Shi Z, Miao X. Novel crosstalk between ethylene- and jasmonic acid-pathway responses to a piercing-sucking insect in rice. New Phytol. 2020. (IF10.151)
[13]. Liang C, Zhang X, Wu J, et al. Dynamic control of toxic natural product biosynthesis by an artificial regulatory circuit. Metab Eng. 2020. (IF9.783)
[14]. Zou G, Bao D, Wang Y, et al. Alleviating product inhibition of Trichoderma reesei cellulase complex with a product-activated mushroom endoglucanase. Bioresour Technol. 2021. (IF9.642)
[15]. Gao YQ, Chen JG, Chen ZR, et al. A new vesicle trafficking regulator CTL1 plays a crucial role in ion homeostasis. PLoS Biol. 2017. (IF8.029)
[16]. Xi J, Wu Y, Li G, et al. Mir-29b Mediates the Neural Tube versus Neural Crest Fate Decision during Embryonic Stem Cell Neural Differentiation. Stem Cell Reports. 2017. (IF7.765)
[17]. Li N, Hong T, Li R, et al. Cherry Valley Ducks Mitochondrial Antiviral-Signaling Protein-Mediated Signaling Pathway and Antiviral Activity Research. Front Immunol. 2016. (IF7.561)
[18]. Wang Z, Xi J, Hao X, et al. Red blood cells release microparticles containing human argonaute 2 and miRNAs to target genes of Plasmodium falciparum. Emerg Microbes Infect. 2017. (IF7.163)
[19]. An D, Chen JG, Gao YQ, et al. AtHKT1 drives adaptation of Arabidopsis thaliana to salinity by reducing floral sodium content. PLoS Genet. 2017. (IF5.917)
7. Ordering Information
The products provided by Yeasen are as follows.
Table 1. Ordering Information
|
Product Name |
Product Code |
Specification |
|
10922ES20 |
20 T |
Table 2. Related Products
|
Product Positioning |
Product Name |
Product Code |
Specification |
|
High Fidelity Enzyme 83x Taq |
Hieff Canace™ Plus High-Fidelity DNA Polymerase (Inquiry) |
10153ES60 |
100 U |
|
10153ES76 |
500 U |
||
|
10153ES80 |
1000 U |
||
|
10154ES03 |
1 mL |
||
|
10154ES08 |
5×1 mL |
||
|
Rapid PCR Mix |
10157ES03 |
1 mL |
|
|
10157ES08 |
5×1 mL |
||
|
Antibiotics for Clonal Screening |
60203ES10 |
10 g |
|
|
60203ES60 |
100 g |
||
|
60205ES08 |
5 g |
||
|
60205ES25 |
25 g |
||
|
60205ES60 |
100 g |
||
|
60206ES10 |
10 g |
||
|
60206ES60 |
100 g |