Which enzymes do scientists use to bind a new gene? Needless to say, DNA ligase is included. So why is DNA ligase so important in recombinant DNA? Because DNA ligase is responsible for ligating the target fragment to the vector, which is one of the key elements that determine the success of the experiment. As a kind of DNA ligase, what role does T4 DNA ligase play in molecular cloning experiments? How does it work? The T4 DNA ligase will be thoroughly introduced next.
1. What is T4 DNA Ligase?
2. What is the function of T4 DNA ligase?
3. Yeasen Biotech T4 DNA ligase can be used for NGS adapter ligation
4. A selection guide for Yeasen Biotech T4 DNA ligase
1. What is T4 DNA Ligase?
T4 DNA ligase is an ATP-dependent ligase that catalyzes the ligation reaction between DNA molecules. It mainly forms phosphodiester by linking the 3'-hydroxyl and 5'-phosphate ends. DNA ligases are involved in DNA replication and repair processes in all organisms. The phage-encoded T4 DNA ligase is produced during phage T4 infection of E. coli.
The ligases used in genetic engineering are mainly E. coli DNA ligase and T4 DNA ligase, the latter being more widely used at present. T4 DNA ligase can repair single-stranded nicks on double-stranded DNA, double-stranded RNA, or DNA/RNA hybrid strands to connect two adjacent nucleotides, and plays an important role in DNA repair and recombination.
In the recombinant plasmid construction process, T4 DNA ligase can be used together with restriction enzymes to complete the recombinant plasmid construction experiment. It can catalyze the formation of a phosphodiester bond between the 5'-P end and 3'-OH end of double-stranded DNA and has a good connection efficiency for sticky end connection and blunt end connection.
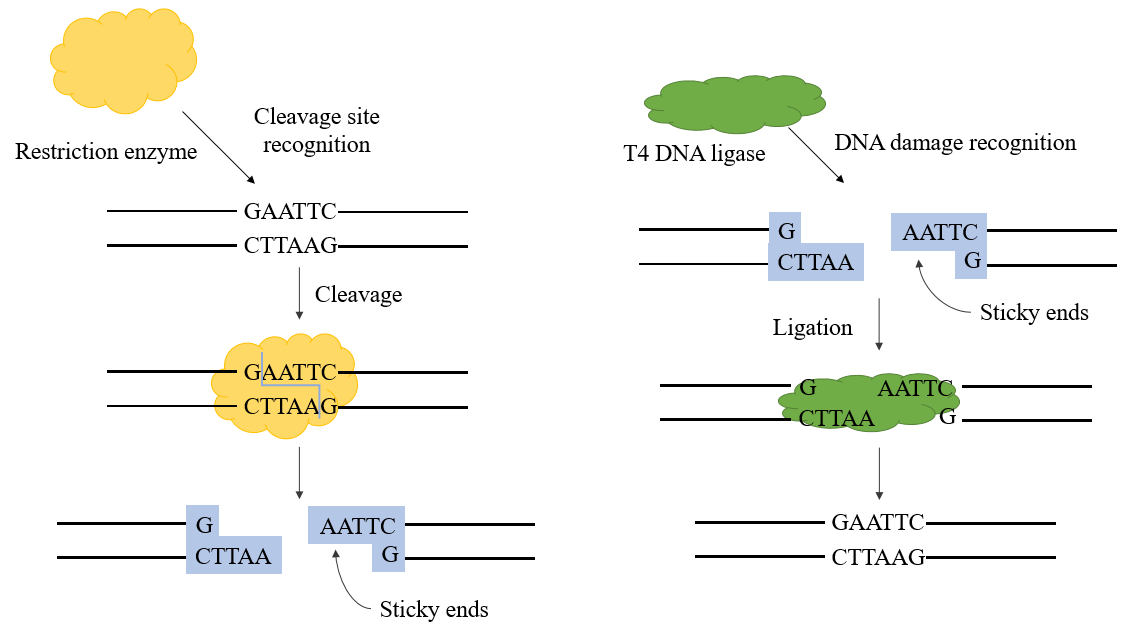
Figure 1. T4 dna ligase mechanism
2. What is the function of T4 DNA ligase?
2.1 Vector Construction
In vector construction experiments, different restriction enzymes can produce different types of ends. For different ends, T4 DNA ligase will have different ligation strategies.
2.1.1 Cloning with restriction enzymes, sticky ends produced by single digest
During the construction of the vector, if the same restriction endonuclease is used to cut the DNA fragment of the target gene and the vector molecule can produce the same sticky end, the T4 DNA ligase can directly carry out the recombination connection. However, because the sticky ends are the same, the target gene can be inserted into the vector in the forward or reverse direction, which will easily increase the workload of screening for correct recombinant clones. Consider using the double-enzyme digestion method for vector construction.
In addition, the cohesive ends of the vector prepared by single enzyme digestion can also be paired, and then phosphodiester bonds are formed between nucleotides under the action of T4 DNA ligase, resulting in the self-ligation of the vector. Using alkaline phosphatase to treat the digested vector can remove the phosphate group at the 5' end of the vector so that the vector cannot complete self-ligation. Thus, under the action of T4 DNA ligase, the vector and the target fragment are connected to complete the construction of the recombinant vector.
2.1.2 Cloning with restriction enzymes, sticky ends produced by double digests
In the process of vector construction, if two restriction enzymes with different sticky ends are used to digest the target fragment and the vector respectively, two different sticky ends can be generated. At this point, T4 DNA ligase can selectively ligate the same sticky ends to ensure that the target fragment is inserted into the vector in the correct direction. When the target fragment and the vector in Figure 2 are digested with EcoR I and BamH I at the same time, the same sticky ends can be connected. There is only one ligation direction between the target fragment and the vector.

Figure 2. Sticky end ligation generated by double-enzyme digestion[1]
2.1.3 Cloning of restriction fragments, blunt end
Some restriction endonucleases can also generate blunt ends during enzymatic cleavages, such as Sma I and others. T4 DNA ligase can directly form a phosphodiester bond between the vector and insert, and there is no need for pairing between bases. However, this method has low ligation efficiency and is prone to vector self-ligation. Generally, the blunt ends can be converted into sticky ends and then ligated. For example, adding complementary poly A and poly T bases to the ends of the target fragment and the vector and artificially sticky complementary ends respectively improve the connection efficiency by terminal deoxynucleotidyl transferase.
2.1.4 TA cloning
The T vector used in TA cloning has a T -overhang at the 3' end. When the DNA sequence of the target fragment is unclear, the target gene fragment can be connected to the T vector by TA cloning, and the target gene can be determined by sequencing. The Taq DNA polymerase used in PCR has terminal transferase activity and can add a nucleotide "A" to the 3' end of the DNA fragment. T4 DNA ligase can directly connect the product amplified by Taq DNA polymerase to the T vector, and the PCR amplified product can achieve the purpose of efficient cloning without adding artificial adapters.
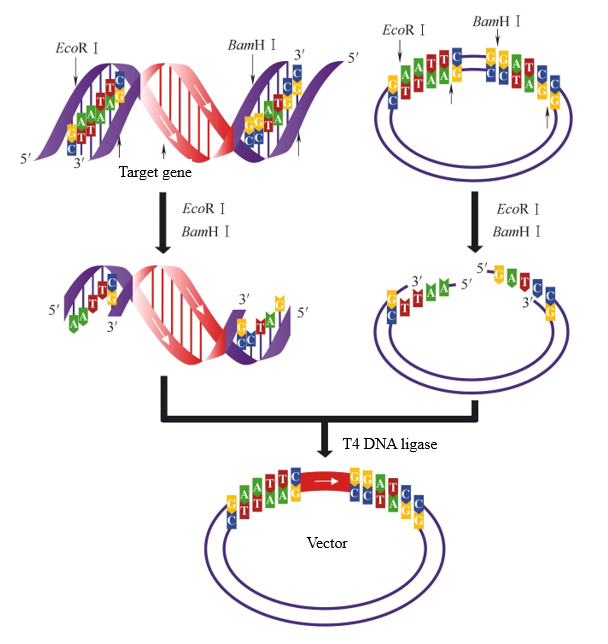
Figure 3. The workflow of TA cloning[2]
2.2 NGS adapter ligation
During the construction of the next-generation sequencing library, it is necessary to connect the artificial adapter to the PCR product before it can be fixed on the flow cell on the sequencing chip to complete the sequencing. TA cloning ligation linker library building is a very common technical means, and its principle is similar to the above-mentioned TA cloning. After the DNA fragment to be sequenced is phosphorylated at the 5' end and "A" is added at the 3' end, it is complementary and paired with the adapter with the "T" sticky end. The complete double strand is then formed and sequenced by the machine.
During TA ligation, different sample types or the complexity of the nucleic acid fragment structure will affect the efficiency of the ligation, so the adapters of different platforms will also have an impact on the final library result.
For example, the Bubble adapter of the MGI platform has a special secondary structure and requires very high ligation efficiency for T4 DNA ligase, and the reduction of ligation efficiency directly affects the output of the library.
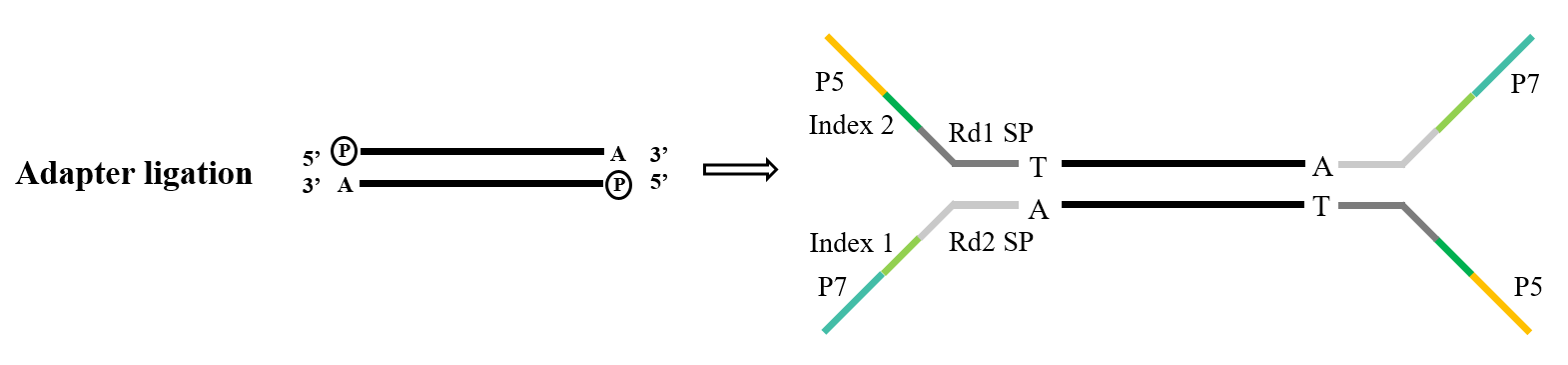
Figure 4. General adapter ligation process
3. Yeasen Biotech T4 DNA ligase can be used for NGS adapter ligation
Yeasen Biotech specially developed Fast T4 DNA ligase for the ligation of DNA fragments and adapters in the process of NGS library construction. The enzyme has efficient ligation ability, not only fast connection speed but also compatible with various types of samples, which is more advantageous for the connection of nucleic acid fragments with complex structures. At present, it has been verified by high-throughput sequencing of a large number of customers. For the connection of Bubble adapters on the MGI platform, excellent sequencing quality can also be obtained.
3.1 Yeasen Biotech Fast T4 DNA ligase with the ultra-high ligation efficiency
Use Yeasen Biotech Fast T4 DNA ligase to construct libraries with different adapter types. The sample is a 170 bp cfDNA mimic, and the Agilent 2100 library is used to detect the results. ①Unconnected adapter product; ②Single-end adapter product; ③Double-end adapter product; ④Residual adapter. From the results, it can be seen that the ligation efficiency of single-ended and double-ended adapters is very high.
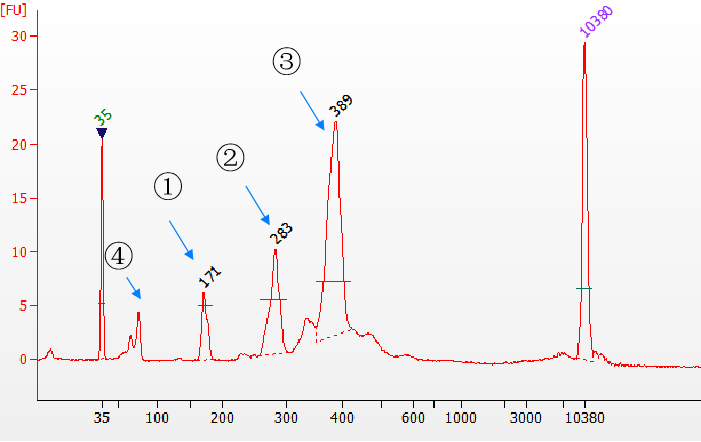
Figure 5. Different types of ligation products detected by the Agilent 2100
3.2 Yeasen Biotech Fast T4 DNA ligase with excellent library yield
Using Fast T4 DNA ligase for different types of library construction, compared with other T4 DNA ligases, library yields are better.
Table 1. Libraries yield of different kinds of samples
|
Types of samples |
Intestinal microbiota gDNA |
cfDNA |
FFPE HD200 gDNA |
|||
|
T4 DNA ligase (same unit) |
Yeasen |
N* |
Yeasen |
N* |
Yeasen |
N* |
|
Input DNA (ng) |
10 |
10 |
50 |
|||
|
Amplification cycle number |
10 |
10 |
8 |
|||
|
Average yield (μg) Illumina platform |
3.3 |
2.8 |
2.7 |
2.2 |
3 |
2.5 |
|
Average yield (μg) MGI platform |
2.7 |
0.9 |
2.0 |
0.7 |
2.3 |
0.8 |
4. A selection guide for Yeasen Biotech T4 DNA ligase
Yeasen is a biotechnology company engaged in the research, development, production, and sales of three major biological reagents: molecules, proteins, and cells. In addition to Fast T4 DNA ligase, Yeasen Biotech also has Novel T4 DNA ligase and Quick T4 DNA ligase to choose from. You can choose them based on applications displayed in the following table:
Table 2: Related products
|
Product positioning |
Product name |
Cat# |
Application |
|
Universal |
Quick T4 DNA Ligase (400 U/µL) (Inquire) |
10301ES |
Molecular cloning, NGS library construction, etc. |
|
High ligation efficiency and low host E. coli residues |
Fast T4 DNA Ligase (400 U/µL) (Inquire) |
10299ES |
NGS library construction, especially suitable for pathogen detection, NIPT detection, etc. |
|
High sensitivity |
Novel T4 DNA Ligase (400 U/µL) (Inquire) |
10298ES |
NGS library construction, especially suitable for library construction of cfDNA samples. |
References
[1] W. Yuan. Gene Engineering[M]. Chemical Industry Press, 2019.
[2] Clark D P, Pazdernik N J, Mcgehee M R. Cloning Genes for Synthetic Biology - ScienceDirect[J]. Molecular Biology (Third Edition), 2019:199-239.
[3] Tomkinson A E, Vijayakumar S, Pascal J M, et al. DNA Ligases: Structure, Reaction Mechanism, and Function[J]. Chemical Reviews, 2006, 106(2):687-699.
[4] Shuman S. DNA Ligases: Progress and Prospects[J]. Journal of Biological Chemistry, 2009, 284(26):17365-17369.