Acute pancreatitis (AP) is an imbalance between the digestive enzymes and stress signals produced by the pancreas through various mechanisms, resulting in the inflammatory response of the pancreas to its own digestion and surrounding tissues. Symptoms of AP include specific and recurrent abdominal pain, accompanied by fever and acute inflammatory response, divided into mild acute pancreatitis (MAP) and severe acute pancreatitis (SAP). AP is mild discomfort at first, and then causes chronic organ dysfunction to develop into SAP, with a mortality rate as high as 20% to 30%. The clinical treatment of AP is mainly to inhibit the inflammatory response, so as to reduce the mortality rate. AP animal model is the best way to study the pathogenesis of pancreatitis and explore clinical treatment. So what are the commonly used AP animal model preparation methods? What is the modeling mechanism of Caerulein?
1. What are the commonly used AP animal model preparation methods?
2. What is the modeling mechanism of Caerulein?
3. Protocol of Caerulein induced AP model
4. Ordering Information
1. What are the commonly used AP animal model preparation methods?
An ideal animal model should have the advantages of simple construction, easy replication, controllable process, and good simulation of human diseases. The methods used to construct animal models of AP include basic amino acids, secretagogues, alcohol, and retrograde injection of bile acids, etc. To date, caerulein remains the most widely used compound to induce AP (CER-AP) in rodents.
1.1 Secretagogue-induced AP animal model
Normal pancreatic metabolism is related to the physiological concentration of secretagogue. AP is due to the secretion of secretin in large quantities, which in turn promotes the secretion of pancreatic digestive enzymes. A large amount of pancreatic digestive enzymes leads to pancreatic self-digestion. The most commonly used secretagogue is cerulein, a 14 amino acid residue decapeptide cholecystokinin analog that stimulates gastrointestinal hormone secretion. Cerulein is usually used to induce mild AP model, and the construction method is usually intraperitoneal injection of 50 μg/kg cerulein, generally 6 to 7 injections in total, with an injection interval of 1 hour. Generally, after 7 injections, the pancreas may experience obvious edema, inflammatory cell infiltration, and pancreatic tissue necrosis. Caerulein induction is fast, the model has high repeatability, wide applicability, and strong stability, and is often used to evaluate systemic changes in AP.
1.2 Basic amino acid induced AP animal model
Under physiological conditions, the pancreas absorbs a large amount of amino acids and synthesizes them into pancreatic enzymes, but the accumulation of high-concentration amino acids will damage the exocrine glands of the pancreas and induce AP. L-arginine is a basic amino acid widely used to induce AP animal models, and the usual method is intraperitoneal injection of 2.5-5.0 g/kg L-arginine. Higher doses of L-arginine can cause high mortality, lower doses can delay the onset of AP, and repeated administration can promote pancreatic tissue necrosis. L-arginine induces AP model has no clear mechanism of action, it may be because L-arginine induces a large number of oxygen free radicals, destroys the stability of zymogen granules, leads to the release of a large number of digestive enzymes, and increases NO and inflammatory mediators Level. And L-arginine can cause irreversible damage to pancreatic mitochondria, and mitochondrial dysfunction plays an important role in the pathogenesis of AP. The L-arginine-induced AP model has high reproducibility and applicability, is widely used, and has strong stability. However, its disadvantage is that the clinical relevance is low, and only a few cases have been reported that L-arginine causes AP in humans. In addition to L-arginine, L-lysine can also be used to induce AP animal models, which is related to its selective damage to pancreatic mitochondria leading to apoptosis.
1.3 Alcohol-induced AP animal model
Heavy drinking is one of the main causes of human AP, but animal experiments have found that alcohol alone does not cause significant edema, inflammatory cell infiltration, and pancreatic tissue necrosis in experimental animals, but can only cause pancreatic microcirculation disturbance. Therefore, alcohol-induced pancreatitis does not rely solely on ethanol stimulation, and may also require the combined action of other factors to stimulate excessive secretion of pancreatic tissue. Some studies have combined the application of ethanol and palmitoleic acid, which can cause AP in experimental animals after intraperitoneal injection. The mechanism may be that ethanol and palmitoleic acid are combined in the pancreas to generate fatty acid ethyl ester, which is an important pathogenic factor leading to AP. Although the alcohol-induced pancreatitis model is low in cost and easy to operate, it needs to be combined with exocrine overstimulation.
1.4 Animal model of AP induced by bile salt injection into pancreaticobiliary duct
This model usually uses sodium taurocholate to induce the AP model. The induction method is to open the abdomen and incise the anterior wall of the duodenum, and inject bile salts from the ampulla of the duodenum into the pancreatic duct. Sodium taurocholate-induced AP is one of the common induction methods, which simulates the obstruction of the lower common bile duct and has high clinical relevance, similar to the pathogenesis of biliary pancreatitis. Most of these models have multiple organ failure, which are suitable for the study of local and systemic complications of AP, and can also provide research tools for the targeted therapy of SAP. However, such models are characterized by high mortality rates, and it is impossible to evaluate the impact of injection-induced changes in pancreaticobiliary duct pressure on the condition.
2. What is the modeling mechanism of Caerulein?
Caerulein (Ceruletide, Cerulein), a decapeptide and a potent cholecystokinin receptor agonist, was first isolated from skin extracts of the Australian green tree frog (Litoria caerulea), similar chemically and biologically to the human gastrointestinal hormones cholecystokinin-pancreozymin (CCK) and gastrin II. Caerulein stimulates the excessive secretion of pancreatic acinar cells (PAC), hinders the separation of trypsinogen and lysosomal hydrolase, and activates trypsinogen in a cathepsin B-dependent manner. Activated trypsinogen further promotes activation and secretion of pancreatic proteases, leading to inflammation and cellular damage to PACs.
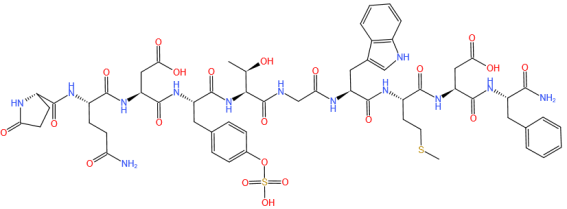
Figure 1. Caerulein Chemical Structure (CAS NO.17650-98-5)
This model has been successfully applied in rats, mice, dogs and Syrian hamsters etc, and is suitable for studying impaired autophagy, pathological calcium signaling, and ER stress, in which AP is characterised by variation in the amount of caerulein, by adjusting the number of injections, resulting in differences in degree of severity.
3.Protocol of Caerulein induced AP model
3.1 Preparation of cerulein solution
Dissolve 1mg of cerulein in 1mL of saline. Sterilize with 0.22μm membrane filter and store at -20°C. Thaw before use and dilute with saline to 5 μg/mL or 10 μg/mL working solution.
3.2 Animal Preparation
Cerulein can induce AP in most animals, mainly used in rodents (such as rats and mice).
3.3 Drug administration
The AP induced by cerulein is mostly oedematous type, which is mostly used in the study of mild AP (MAP) and the conversion of MAP to SAP. For SAP research, caerulein is often combined with other compounds to achieve increased severity of AP, for example, lipopolysaccharide (LPS).
Animals were fasted but could drink freely 12 hours before surgery. Intraperitoneal injection with Caerulein (20-100 μg/kg, usually 50 μg/kg) every hour for 7 h, followed by one injection of 10 mg/kg LPS.
3.4 Model evaluation
⑴ Serum amylase and lipase activities increased.
⑵ Elevated NO concentration in serum.
⑶ Pancreatic pathology: pancreatic tissue edema with vacuoles, inflammatory cell infiltration, periductal and focal acinar cell necrosis.
⑷ Increased expression of inflammatory factors in serum and pancreatic tissue.
3.5 Yeasen biotech Launching product Cat#60321ES Caerulein
Published Data
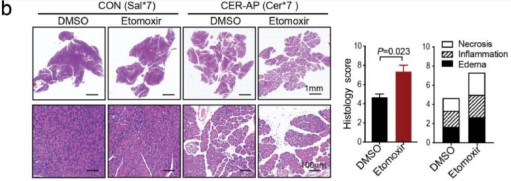
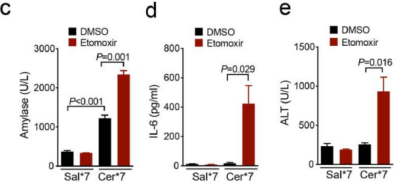
Figure 2. Balb/c mice were administrated (i.p.) hourly of 50 μg/kg caerulein (Yeasen Biotech) dissolved in normal saline for 7 times (Cer*7), and LPS (10 mg/kg) superimposed on a Cer*7 regimen right after the last injection of caerulein to induce necrotising SAP. (b) Representative pancreatic H&E images and histology score. (c-e) Serum levels of amylase (c), IL-6 (d) and ALT (e) from the indicated groups. (PMID: 35339899, IF: 5.736)
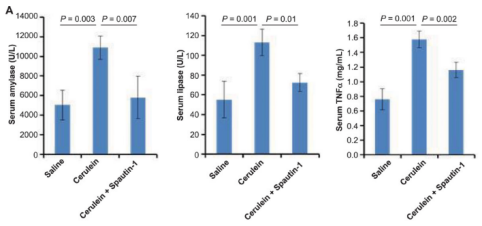
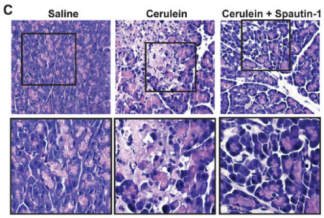
Figure 3. Female KunMing mice (weighed 20-25g) were administrated (i.p.) hourly of 50 μg/kg caerulein (Yeasen Biotech) for four times to induce AP. (A) Serum amylase, lipase and TNFα levels. (C) Representative pancreatic H&E images. (PMID: 27579473, IF: 4.096)
4. Ordering Information
The products provided by Yeasen are as follows.
Table 1. Ordering information
|
Product Name |
Catalog # |
Size |
|
Caerulein (Inquire) |
60321ES03 |
1 mg |
References
[1] Zhang L, et al. Ketogenesis acts as an endogenous protective programme to restrain inflammatory macrophage activation during acute pancreatitis. EBioMedicine. 2022 Apr;78:103959. PMID: 35339899
[2] Xiao J, et al. Spautin-1 Ameliorates Acute Pancreatitis via Inhibiting Impaired Autophagy and Alleviating Calcium Overload. Mol Med. 2016 Oct;22:643-652. PMID: 27579473